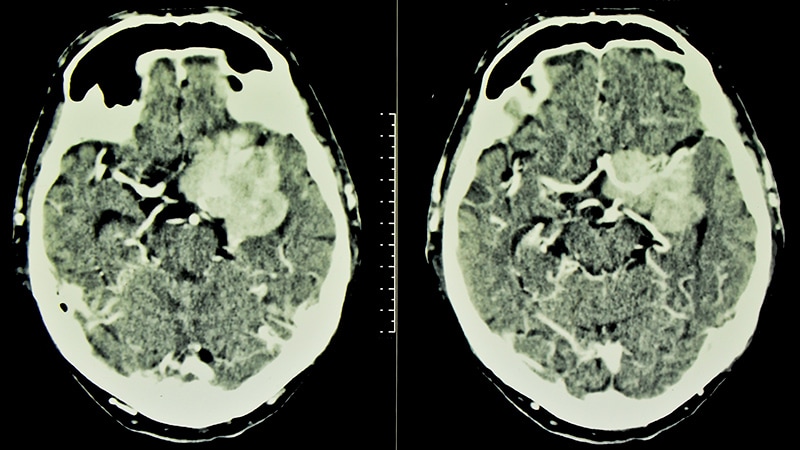
New details confirm an increased risk of developing intracranial meningiomas after prolonged use of the hormonal product cyproterone acetate.
Cyproterone acetate is a synthetic progestogen and potent anti-androgen that has been used in the treatment of hirsutism, alopecia, early puberty, amenorrhea, acne, and prostate cancer, and has also been combined with an estrogen in hormone replacement therapy.
The new findings were published online today in the BMJ.
The primary analysis showed that among women using cyproterone acetate, the rate of meningiomas was 23.8 per 100,000 person years vs 4.5 per 100,000 in the control group. After adjusting for confounders, cyproterone acetate was associated with a sevenfold increased risk of meningioma.
These were young women — the mean age of participants was 29.4 years and more than 40% of the cohort were younger than 25 years. The initial prescriber was a gynecologist for more than half (56.7%) of the participants, and 31.6% of prescriptions could correspond to the treatment of acne without hirsutism; 13.1% of prescriptions were compatible with management of hirsutism.
"Our study provides confirmation of the risk but also the measurement of the dose–effect relationship, the decrease in the risk after stopping use, and the preferential anatomical localization of meningiomas," lead author Alain Weill, MD, EPI-PHARE Scientific Interest Group, Saint-Denis, France.
"A large proportion of meningiomas involve the skull base, which is of considerable importance because skull base meningioma surgery is one of the most challenging forms of surgery and is associated with a much higher risk than surgery for convexity meningiomas," he told Medscape Medical News.
Cyproterone acetate products have been available in Europe since the 1970s under various trade names and dose strengths (1, 2, 10, 50, and 100 mg), and marketed for various indications. These products are also marketed in many other industrialized nations, but not in the United States or Japan.
The link between cyproterone acetate and an increased risk of meningioma has been known for the past decade, and information on the risk of meningioma is already included in the prescribing information for cyproterone products.
Last year, the European Medicines Agency (EMA) strengthened the warnings that were already in place and recommended that cyproterone products with daily doses of 10 mg or more be restricted because of the risk of developing meningioma.
"The recommendation from the EMA is a direct consequence of our study, that was sent to the EMA in summary form in 2018 and followed up with a very detailed with a report in summer 2019," said Weill. "In light of this report, the European Medicines Agency recommended in February 2020 that drugs containing 10 mg or more of cyproterone acetate should only be used for hirsutism, androgenic alopecia, and acne and seborrhea once other treatment options have failed, including treatment with lower doses."
Weill pointed out that two other epidemiologic studies have assessed the link between cyproterone acetate use and meningioma and showed an association. "Those studies and our own study are complementary and provide a coherent set of epidemiological evidence," he told Medscape Medical News. "They show a documented risk for high dose cyproterone acetate in men, women, and transgender people, and the absence of any observed risk for low dose cyproterone acetate use in women."
Strong Dose–Effect Relationship
For their study, Weill and colleagues used data from the French administrative healthcare database. Between 2007 and 2014, 253,777 girls and women aged 7-70 years had begun using cyproterone acetate during that time period.
All participants had received at least one prescription for high dose cyproterone acetate and did not have a history of meningioma, benign brain tumors, or long-term disease. They were considered to be exposed if they had received a cumulative dose of at least 3 g during the first 6 months (139,222 participants) and very slightly exposed (control group) when they had received a cumulative dose of less than 3 g (114,555 participants).
Overall, a total of 69 meningiomas were diagnosed in the exposed group (during 289,544 person years of follow-up) and 20 meningiomas in the control group (during 439,949 person years of follow-up). All were treated by surgery or radiotherapy.
When the analysis was done according to the cumulative dose, it showed a dose–effect relation, with a higher risk associated with a higher cumulative dose. The hazard ratio was not significant for exposure to less than 12 g of cyproterone acetate, but it jumped rapidly jumped as the dose climbed: the hazard ratio was 11.3 for 36-60 g and was 21.7 for 60 g or higher.
In a secondary analysis, the authors looked at the cohort who were already using cyproterone acetate in 2006 (n = 123,997). Women with long-term exposure were also taking estrogens more often (55.5% v 31.9%), and the incidence of meningioma in the exposed group was 141 per 100,000 person years, which was a risk greater than 20-fold (adjusted hazard ratio 21.2.) They also observed a strong dose–effect relationship, with adjusted hazard ratio ranging from 5.0 to 31.1.
However, the risk of meningioma decreased noticeably after treatment was stopped. At 1 year after discontinuing treatment, the risk of meningioma in the exposed group was 1.8-fold higher (1.0 to 3.2) than in the control group.
Weill noted the clinical implications of these findings: clinicians need to inform patients who have used high-dose cyproterone acetate for at least 3 to 5 years about the increased risk of intracranial meningioma, he said.
"The indication of cyproterone acetate should be clearly defined and the lowest possible daily dose used," he said. "In the context of prolonged use of high-dose cyproterone acetate, magnetic resonance imaging screening for meningioma should be considered."
"In patients with a documented meningioma, cyproterone acetate should be discontinued because the meningioma might regress in response to treatment discontinuation and invasive treatment could be avoided," Weill added.
Use Only When Necessary
Weighing in on the research, Adilia Hormigo, MD, PhD, director of neuro-oncology at The Tisch Cancer Institute at Mount Sinai Health System in New York City, noted that "it is well known that there are sex differences in the incidence of meningiomas, as they are more frequent in women than men, and there is an association between breast cancer and the occurrence of meningiomas."
Progesterone and androgen receptors have been found in meningiomas, she told Medscape Medical News, and there is no consensus regarding estrogen receptors. "In addition, hormonal therapy to inhibit estrogen or progesterone receptors has not produced any decrease in meningiomas' growth," she said.
The current study revealed an association between prolonged use of cyproterone acetate with an increased incidence of meningiomas, and the sphenoid-orbital meningioma location was specific for the drug use. "It is unclear from the study if all the meningiomas were benign," she said. "Even if they are benign, they can cause severe morbidity, including seizures."
Hormigo recommended that an MRI be performed on any patient who is taking a long course of cyproterone acetate in order to evaluate the development of meningiomas or meningioma progression. "And the drug should only be used when necessary," she added.
This research was funded by the French National Health Insurance Fund (CNAM) and the Health Product Epidemiology Scientific Interest Group (ANSM-CNAM EPI-PHARE SIG). Weill is an employee of the French National Health Insurance Fund, as are several other coauthors. The other authors have disclosed no relevant financial relationships. Hormigo has disclosed no relevant financial relationships.
BMJ. Published online February 3, 2021. Full text
For more from Medscape Oncology, join us on Twitter and Facebook
"use" - Google News
February 04, 2021 at 06:42AM
https://ift.tt/3rkmGfl
Increased Risk of Meningioma With Cyproterone Acetate Use - Medscape
"use" - Google News
https://ift.tt/2P05tHQ
https://ift.tt/2YCP29R
Bagikan Berita Ini














0 Response to "Increased Risk of Meningioma With Cyproterone Acetate Use - Medscape"
Post a Comment